Below is a flow chart demonstrating the process UIRF takes to commercialize new innovations.
Below is a flow chart demonstrating the process UIRF takes to commercialize new innovations.
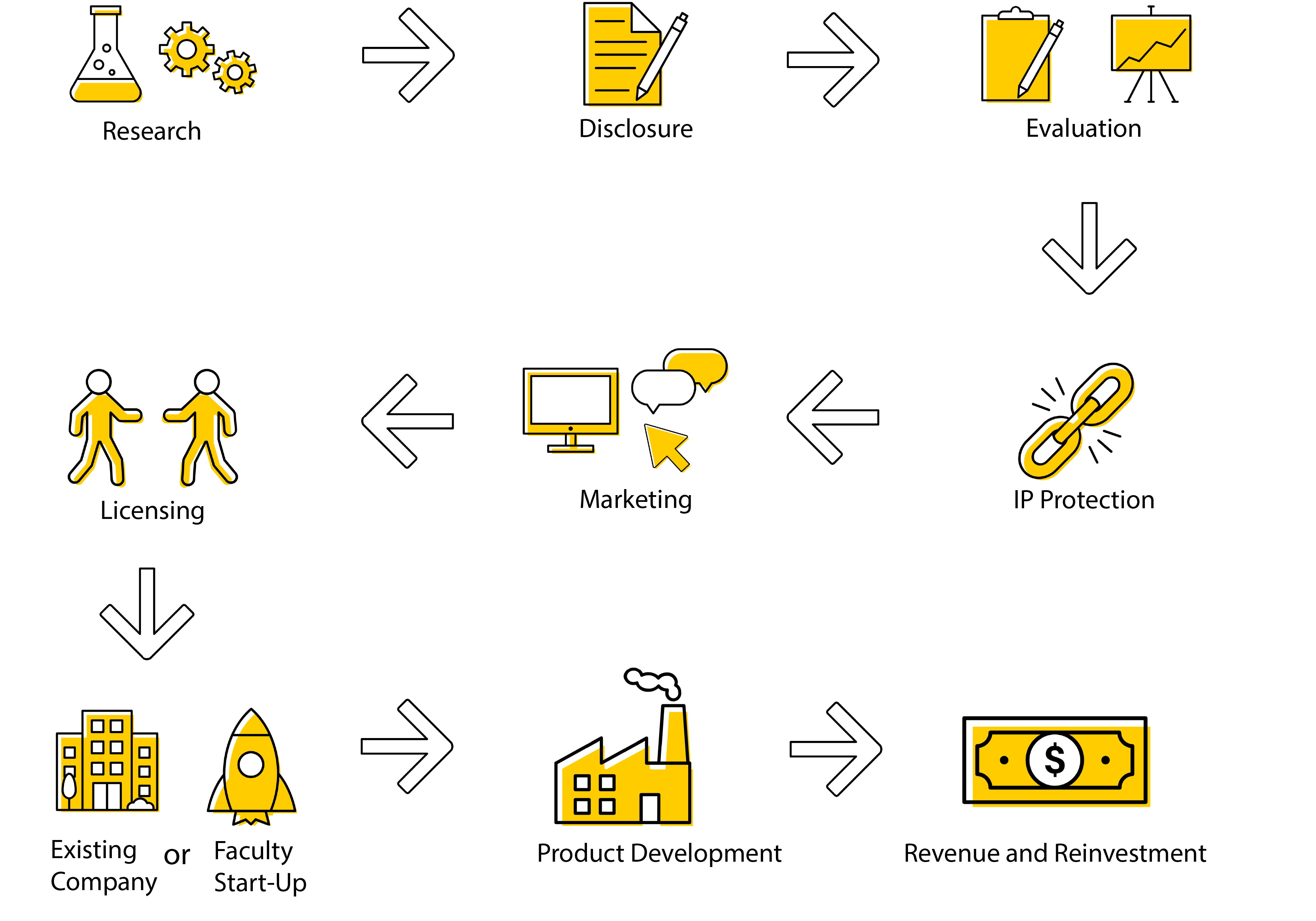
Our Process
Technology developed at the University of Iowa is typically basic research. Additional investment beyond the research, both in dollars and diligent effort, is often needed to move the technology from the university to the marketplace. The first part of this process involves careful review by UIRF and investment in intellectual property protection through patent applications or copyright filings to protect the technology. In addition, UIRF will seek external partnerships to further the commercialization effort for selected technologies. Please review below to learn more or reach out to a licensing staff member.
UIRF Commercialization Process
Research
Innovations emerge from the research you conduct at the University of Iowa. When your research results point to a commercial application, we encourage you to submit an invention disclosure or reach out to the licensing staff
Disclosure
UI faculty, staff, and students are encouraged to disclose their inventions to UIRF as early as possible. If you have any public disclosures (as described below) planned in the near future, it is crucial that you inform us. Public disclosures can include the publication of articles or meeting abstracts, talks or poster presentations at conferences, or discussions with others who are not part of the University of Iowa community. Keep in mind that merely submitting an invention disclosure does not automatically provide any Intellectual Property (IP) protection. If you have excerpts from a grant application or a draft manuscript, you can include those with your disclosure form. If you need help submitting your disclosure, please don’t hesitate to reach out to our licensing staff.
Evaluation
Once you submit an invention disclosure form to UIRF, our licensing staff will review the documents you provided. Your licensing manager will schedule a meeting with you to discuss key aspects of your innovation, and work with you on an IP and commercialization strategy. Following the initial meeting, we will conduct an IP and market review to assess the potential for IP protection and commercial viability of the innovation. It’s important to note that not all disclosed innovations result in patents. Some innovations may be better suited for other forms of IP protection, or they may require additional data or information to strengthen their IP protection.
IP Protection
The patent process, spanning from filing to issuance, can take several years, and not every disclosed invention requires patent protection. When patent protection is appropriate for an invention, we will collaborate with you and any co-inventors. This collaboration involves working with a member of our licensing staff and a patent attorney appointed by UIRF to navigate the patent prosecution process, and UIRF covers the cost for engaging the patent attorney and filing the patent application.
Detailed information on the patent prosecution process can be found here.
Copyright Protection
Copyright protection differs from patent protection. Patents protect inventions, while copyrights protect the creative expression of works. It’s important to note that copyright does not protect the ideas behind a work. While patents have inventors, copyrights have authors. However, authorship under copyright law is different from authorship in academic contexts. An author is anyone who creates the work, not necessarily the person who conceived the ideas. For example, a student who writes code for a program at the request of a non-coding supervisor is considered the author of the work. Copyright authorship can be complex, and UIRF can help you and your colleagues determine who should be included as an author of the copyright.
Unlike patents for inventions, copyright on a creative work exists the moment the work is created. For example, as soon as the student in the previous example finishes writing the computer program, it is covered by copyright protection. There are limited circumstances under which UIRF might want to register the existing copyright with the U.S. Library of Congress. However, in most cases, UIRF files a registration only when a company licensee requests it.
Research Tools
Most cell lines, antibodies, mouse models, and other research tools may not require patent protection to proceed with commercialization. UIRF protects these tools through the terms of its contracts with the companies using or selling them. In some cases, patent protection may be available and useful for a particular tool, and we can discuss whether this is the case for your tool (sometimes, patenting a tool can make it cost-prohibitive to license it to a company and sometimes having a patent shortens the timeframe over which we can collect payment on the tool).
Marketing
After taking the necessary steps to evaluate the IP rights and identifying the innovation’s commercial potential, we market the innovation to potential industry partners. This process involves providing suggestions and feedback to the licensing manager working with you as they draft marketing materials and build a list of potential industry partners to contact. Your feedback is crucial to ensure that we highlight the key features and benefits of the innovation and describe it in simple language that can be easily understood by potential industry partners.
Our primary objective is to find a company interested in obtaining a license for innovation. This involves identifying potential industry partners who see value in the invention and are willing to invest in its further development and commercialization. However, it’s important to note that the marketing process is often a dialogue. We value feedback from these companies on what additional data or results would make the invention more attractive for licensing. This feedback can guide your future research and development efforts. In some cases, companies may be interested in sponsoring research or other forms of collaboration to help advance innovation to a stage where licensing becomes a viable path forward. This iterative process of engagement and feedback is crucial in bridging the gap between academic research and commercial application.
Active involvement from inventors significantly improves the chances of finding a licensee. Once interested companies are identified, you of course are the best person to describe the details of the invention and its technical advantages. Therefore, we will coordinate a meeting in which you will discuss the invention in greater detail. Typically, companies are most interested in licensing if their researchers and business development staff understand and feel confident in the research the inventors have conducted at the University.
When discussing an invention with a potential industry partner, it may be necessary for UIRF and the other party to sign a Confidential Disclosure Agreement (CDA) before any discussions. This agreement helps ensure that any shared confidential information is used and disclosed appropriately. If the other party shares its own confidential information, just remember to keep it separate, share it only with those who need to know and understand the obligations, return or destroy it when the agreement ends, and avoid using it for new projects or public disclosures without permission. We can walk you through the terms of your CDA so that you understand our obligations and the company’s responsibilities.
Licensing
Companies interested in using or selling a product or service based on your innovation can enter into a license agreement with UIRF. The agreement spells out the financial and legal terms under which UIRF grants the company rights to commercially use this IP to benefit society and the general economy.
Alternatively, companies may elect to obtain an option agreement. An option agreement provides a company a time-limited right (usually 6 months to a year) to obtain a full license agreement. Options are typically used in instances where the company would like to do further research and development to evaluate the innovation prior to committing to a full license agreement.
Technologies can be licensed by an existing company, or a start-up company created to commercialize the innovation. Faculty interested in starting a company can work closely with the UI Ventures team to form the new company and determine the best path forward for commercialization. Once the company is formed, UIRF can then option or license the innovation to the start-up.
(If you are interested in learning about UI technologies available for licensing, please visit our portfolio or contact the UIRF licensing staff.)
Product Development
The University of Iowa is committed to making sure innovations are ultimately made available for the public good and benefit of society. In addition, many grants, such as those from federal institutions and many non-profit organizations, require that innovations developed using the funding are made available to the public. To address this, license agreements will have several milestones that a company must meet during the commercialization period to ensure that the UI’s mission and grant compliance is achieved. As most University innovations are disclosed at an early stage, development can be a lengthy process, and it may take many years until the innovation is developed into a product that reaches the market.
Revenue and Reinvestment
License agreements contain financial terms that are designed to promote innovation development and generate revenue. When revenue comes in, UIRF first recovers any outstanding expenses for patent costs, and UIRF may need to make payments to other institutions if, for example, you have co-inventors from another university. The remaining revenue is shared among the inventors, the inventors' departments, and colleges, the “Research Enrichment Fund” managed by the Vice President of Research, and UIRF. Specific information on revenue distribution can be found in the UI IP Policy.